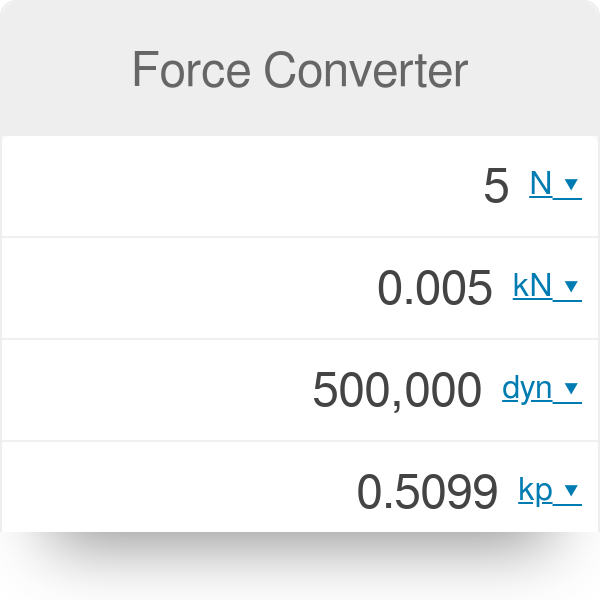
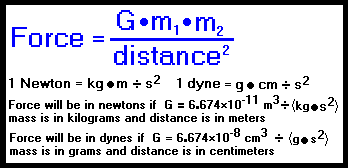
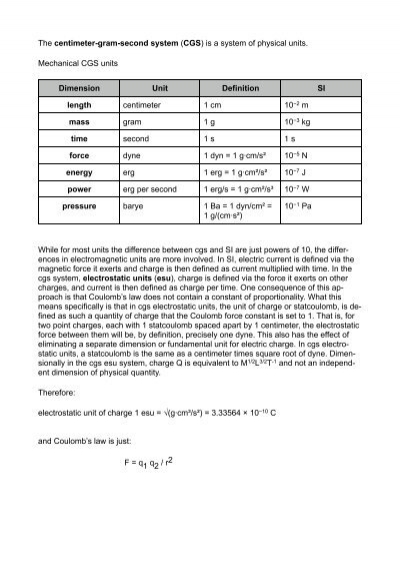
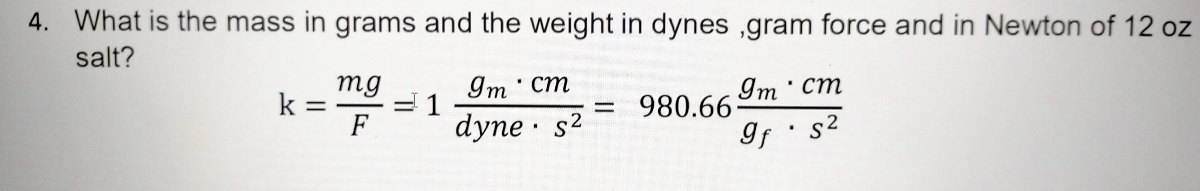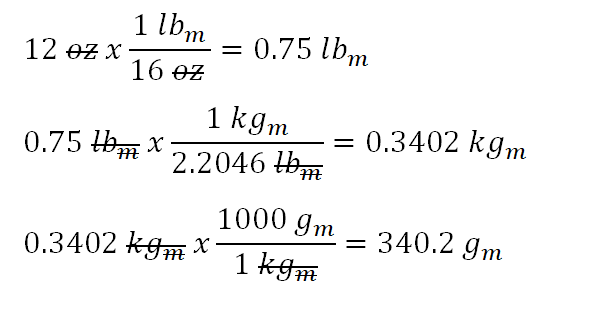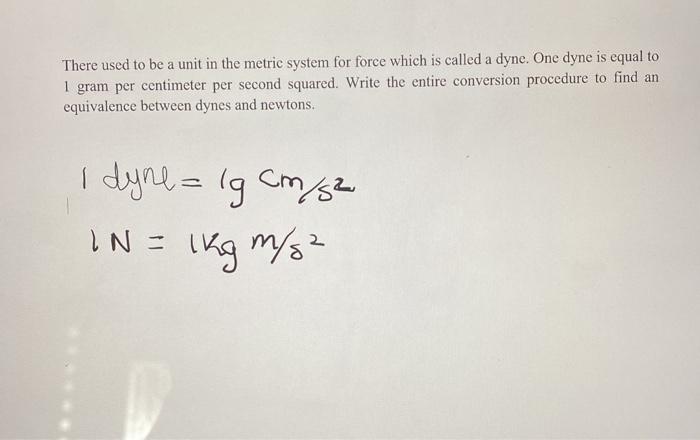Activity 1: solve for size and speed of a drop.. Useful units for calculations Remember: dyne = 1 g cm/s 2 (force in cm-g-s units) = Newtons. Using. - ppt download

The Undertale fandom wiki says Undyne's name came from "Undine, a womanly water nymph from Greek lore." But what if its also a play on this? A "dyne" is a very very

Goals for Chapter 4 To understand the meaning of force in physics To view force as a vector and learn how to combine forces To understand the behavior. - ppt download

A force of 72 dyne is inclined to the horizontal at an angle of 60°. Find the acceleration in a mass of 9 g, which moves in a horizontal direction. - Find 4

Question Video: Finding the Rate of Change of the Mass of a Ball as It Moves through a Dusty Medium | Nagwa